
Teflon is a thermoplastic polymer made from tetrafluoroethylene polymerization and is in solid form.
Teflon has different types: PTFE, FEP, ETFE, and PVDF.
The chemical PTFE is known commercially as Teflon, given by DuPont Company.
Tetrafluoroethylene polymerization yields the thermoplastic polymers Teflon and PTFE, which exist in solid form.
Therefore, PTFE is the scientific name, and Teflon is the Trademark name for the same material.

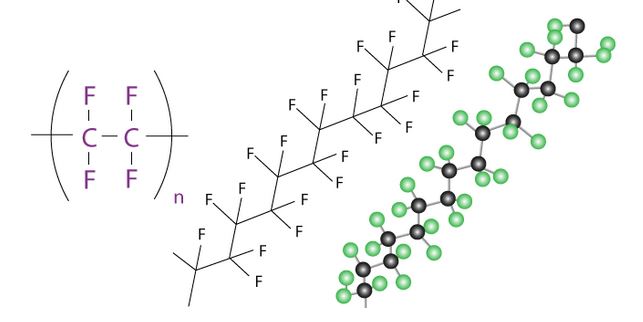
Elements making PTFE and Teflon
PTFE and Teflon have the same chemical element since they refer to the same material.
PTFE and Teflon have the following elements:
Fluorine
Teflon and PTFE have a lot of fluorine atoms in them.
The carbon chain is encased in a protective sheath of fluorine atoms, resulting in a chemically inert, relatively dense molecule with extremely potent carbon-fluorine interactions.
Carbon
Each carbon atom is connected to two other carbon atoms in a lengthy chain-like arrangement of carbon atoms.
Physical Properties
Since Teflon and PTFE mean the same material, the following are their physical properties:
- Appearance
Both polymers are white and in solid form.
- Density
The density of PTFE and Teflon is approximately 2200 kg/m3.
- Smooth and non-stick
PTFE and Teflon have smooth surfaces because of the fluorine surrounding their molecules. Because of these fluorine atoms, Teflon and PTFE resist practically all other materials sticking.
- Low friction
Their low coefficient of friction is contributed by the fluorine coating on the two polymers.
- Melting point
They have a high melting point of up to 327 °C
- Electrical insulation
PTFE and Teflon are not easily porous to electrical energy and can endure high volts without degrading.
- Hydrophobicity
They are water-resistant polymers.
Chemical Properties
The chemical properties of PTFE and Teflon are similar; they include the following:
- Inertness
PTFE and Teflon are exceptionally resilient when attacked chemically by bases, acids, and other corrosive chemicals.
Most solvents do not affect them, and even potent acids and bases don’t readily react with them.
- Resistant to degradation
Due to the presence of carbon atoms and fluorine, PTFE and Teflon have excellent resistance to degradation.
- Reactivity
These polymers are typically non-reactive and hence do react with other compounds.
- Chemical resistance
They are very resistant to bases, acids, and caustic chemicals attacking them chemically.
Mechanical Properties

Teflon and PTFE show similar mechanical properties, which include the following:
- Elongation
PTFE and Teflon have a high extension of up to 500%.
- Compressibility
These polymers have low compressibility as a result of their molecular structure.
Under very high temperatures, PTFE and Teflon cannot change their volume and shape.
- Tensile strength
They have a high tensile strength of approximately 10-43MPa.
- Versatility
Because of their molecular composition, they are adaptable and can flex or bend without deforming.
- Hardness
These polymers have very low hardness as they are soft.
Advantages
Teflon and PTFE’s particular properties impact their advantages and are considered for their various applications.
The following are the advantages of PTFE and Teflon:
- Resistant to chemicals
They are highly resistant to water, solvents, bases, acids, chemicals, and other corrosive substances.
- Electrical insulation
PFOA, just like Teflon, is an excellent electrical insulator as it can endure high volts without damage and is not easily permissible to electric energy.
- Non-stick and smooth
They are very smooth and not sticky hence preferred for non-stick applications such as cookware.
- Low friction surface
Both have a very low friction coefficient and are preferred for engineering applications.
- Resistant to high temperatures
They are suitable for use even in high temperatures as they can withstand up to 260°C without damage.
- Resistant to the harsh environment and weather conditions
The polymers can withstand extreme weather conditions and are considered primarily for outdoor applications.
Disadvantages
Despite PTFE and Teflon having excellent benefits which make them suitable for their applications, they also have their limitations which include the following:
- Cost
Polymers are considerably costly because of processing and manufacturing.
- Limited high-pressure applications
They are inappropriate for high-pressure applications as their compressive strength is relatively low.
- Non-decaying
They can stay for very long in the environment without damage or decay because they are non-biodegradable.
- Bonding difficulty
Teflon and PTFE have low surface energy and do not quickly bond with other materials.
- Limitation of color options
They are white and hence may not be suitable if they are needed in other color options.
Applications
The mechanical, physical, and chemical properties of PTFE and Teflon make them suitable for various applications in the industry.
The following are the applications of PTFE and Teflon:
- Automotive industry
They are used in power steering and transmission, linings for fuel valve stem seals, gaskets, O-rings, and shaft seals.
- Engineering
They are used in construction components such as pipe coatings, fittings, bearings, seats and plugs, and valve and pump parts.
- Electrical and electronics
They are used to manufacture electrical and electronic components such as circuit boards, electrical insulation, wire insulation, and semiconductors.
- Medical applications
Polymers are used to make medical implants such as heart patches, replacement ligaments, and vascular garments.
- Non-stick coatings
Surfaces for cookware, bakeware, and food processing equipment are coated with PTFE and Teflon.
Conclusion
PTFE and Teflon are the same chemical compounds or materials.
Both are synthetic polymers with the same chemical composition and physical, mechanical, and chemical characteristics.
The difference between the two is that Teflon is the Trademark name for PTFE, owned by a company known as DuPont.
More Resources:
PTFE Applications – Source: HANSA
Teflon – Source: Wikipedia
PTFE Manufacturing Process – Source: HANSA
Filled PTFE – Source: HANSA



